Decoding the FDA Drug Approval Calendar 2024: A Potential Take a look at Potential Breakthroughs and Challenges
Associated Articles: Decoding the FDA Drug Approval Calendar 2024: A Potential Take a look at Potential Breakthroughs and Challenges
Introduction
With nice pleasure, we are going to discover the intriguing matter associated to Decoding the FDA Drug Approval Calendar 2024: A Potential Take a look at Potential Breakthroughs and Challenges. Let’s weave fascinating info and provide contemporary views to the readers.
Desk of Content material
Decoding the FDA Drug Approval Calendar 2024: A Potential Take a look at Potential Breakthroughs and Challenges

The FDA drug approval calendar for 2024 stays unwritten, a dynamic panorama formed by ongoing scientific trials, evolving regulatory pathways, and the unpredictable nature of scientific discovery. Nevertheless, by analyzing present pipelines, rising developments, and previous approval patterns, we will anticipate potential areas of focus and vital challenges dealing with the company within the coming yr. This text provides a potential take a look at the 2024 FDA drug approval calendar, exploring potential breakthroughs and the hurdles they could face.
Predicting the Unpredictable: Methodologies and Limitations
Predicting FDA approvals with certainty is inconceivable. The method is advanced, involving rigorous assessment of scientific trial knowledge, manufacturing processes, and labeling info. Sudden security alerts, incomplete knowledge, or manufacturing points can derail even essentially the most promising candidates. However, a number of elements permit for knowledgeable hypothesis:
- Ongoing Scientific Trials: Publicly obtainable info on scientific trials (e.g., ClinicalTrials.gov) supplies perception into the progress of quite a few drug candidates. Part 3 trial completion typically precedes FDA submission, providing a timeline estimate.
- FDA Steerage Paperwork: The FDA releases steerage paperwork outlining expectations for particular therapeutic areas and drug varieties. These paperwork present clues concerning the company’s priorities and the probability of approval for medicine assembly particular standards.
- Previous Approval Patterns: Analyzing previous approval charges for related medicine and therapeutic areas supplies a historic benchmark, though this isn’t a assure of future efficiency.
- Business Analyst Predictions: Pharmaceutical business analysts typically publish predictions based mostly on their evaluation of scientific trial knowledge and regulatory pathways. These predictions ought to be seen with warning, as they’re topic to bias and uncertainty.
Key Therapeutic Areas to Watch in 2024:
A number of therapeutic areas are poised for vital exercise in 2024, based mostly on present scientific trial pipelines:
-
Oncology: Most cancers stays a serious focus of pharmaceutical analysis and growth. We are able to anticipate approvals for novel focused therapies, immunotherapies (together with CAR T-cell therapies and bispecific antibodies), and mixture therapies addressing unmet wants in numerous most cancers varieties. Particular areas of curiosity embrace lung most cancers, colorectal most cancers, and hematological malignancies. Challenges embrace demonstrating sturdy responses and managing toxicity profiles.
-
Neurology: The event of therapies for Alzheimer’s illness, Parkinson’s illness, and different neurodegenerative problems continues to be a serious precedence. A number of disease-modifying therapies are in superior levels of growth, however the excessive failure charge on this space necessitates cautious optimism. Profitable approvals will seemingly rely on demonstrating clear scientific advantages and addressing potential security issues.
-
Infectious Illnesses: The continuing risk of antibiotic resistance and rising infectious illnesses will drive continued growth of novel antimicrobials and antiviral therapies. Approvals on this space could also be influenced by the pressing public well being want and the supply of other therapy choices.
-
Uncommon Illnesses: The Orphan Drug Act supplies incentives for the event of therapies for uncommon illnesses, leading to a big pipeline of potential approvals. Challenges embrace demonstrating efficacy in small affected person populations and navigating the complexities of scientific trial design.
-
Immunology and Autoimmune Illnesses: New therapies concentrating on particular immune pathways are anticipated, aiming to enhance efficacy and scale back uncomfortable side effects in comparison with current therapies for situations equivalent to rheumatoid arthritis, lupus, and inflammatory bowel illness. Approvals will rely on demonstrating superior efficacy and a good security profile.
Potential Breakthroughs and Challenges:
A number of elements may considerably affect the 2024 FDA approval panorama:
-
Accelerated Approval Pathways: The FDA’s accelerated approval pathway permits for the approval of medicine based mostly on surrogate endpoints, offered they present promise in addressing unmet medical wants. Whereas this could expedite entry to doubtlessly life-saving therapies, it additionally necessitates post-market surveillance to verify scientific profit. The steadiness between accelerating entry and making certain affected person security stays a crucial problem.
-
Actual-World Proof (RWE): The growing use of RWE in regulatory decision-making may affect the approval course of. RWE can complement conventional scientific trial knowledge, offering insights into drug effectiveness and security in various affected person populations. Nevertheless, the standard and reliability of RWE want cautious analysis to make sure its acceptable use in regulatory submissions.
-
Synthetic Intelligence (AI) in Drug Improvement: AI is remodeling drug discovery and growth, accelerating the identification of drug targets and optimizing scientific trial design. The FDA’s strategy to regulating AI-driven drug growth continues to be evolving, and its influence on the 2024 approval calendar stays to be seen.
-
Provide Chain Points: Manufacturing and provide chain disruptions can influence the well timed submission and assessment of recent drug functions. The FDA’s potential to handle these challenges successfully shall be essential for sustaining the integrity of the drug approval course of.
-
Useful resource Constraints: The FDA faces ongoing challenges associated to useful resource constraints, together with staffing shortages and budgetary limitations. These constraints can influence the velocity and effectivity of the drug assessment course of.
Wanting Forward:
The 2024 FDA drug approval calendar shall be formed by the interaction of scientific developments, regulatory insurance policies, and unexpected challenges. Whereas predicting particular approvals stays tough, the developments outlined above recommend a yr of serious exercise throughout a number of therapeutic areas. The success of those efforts will rely on the continued collaboration between researchers, regulators, and healthcare suppliers to make sure the event and approval of protected and efficient therapies that tackle unmet medical wants. Shut monitoring of scientific trial progress, FDA steerage paperwork, and business analyses shall be essential for understanding the evolving panorama of drug approvals in 2024. The yr guarantees breakthroughs, but in addition challenges in navigating the advanced path from laboratory discovery to affected person entry. The FDA’s position in balancing innovation with affected person security shall be paramount in shaping the way forward for medication.


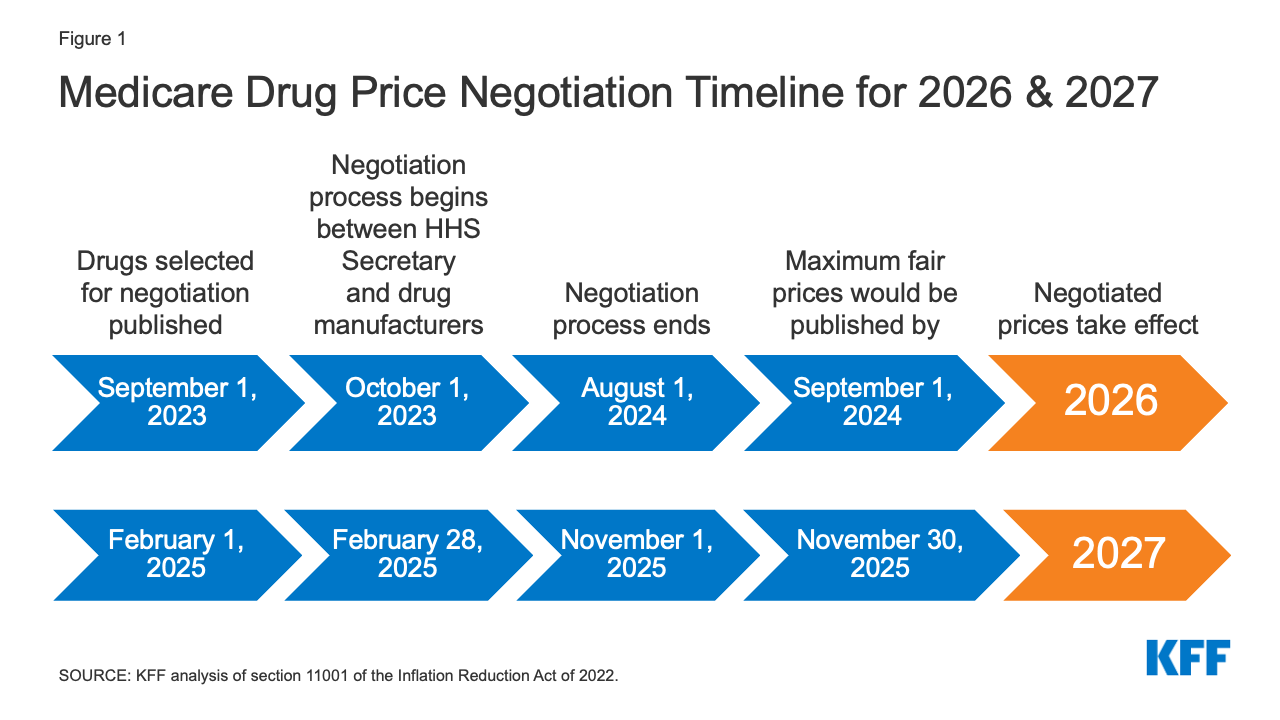
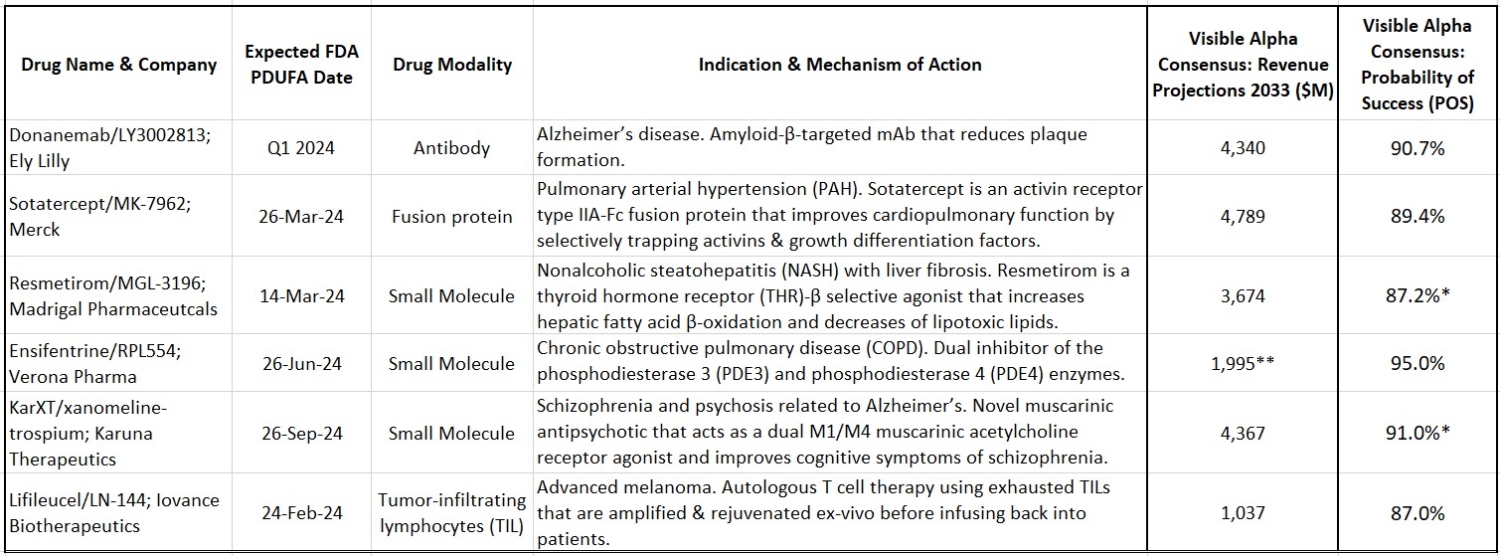
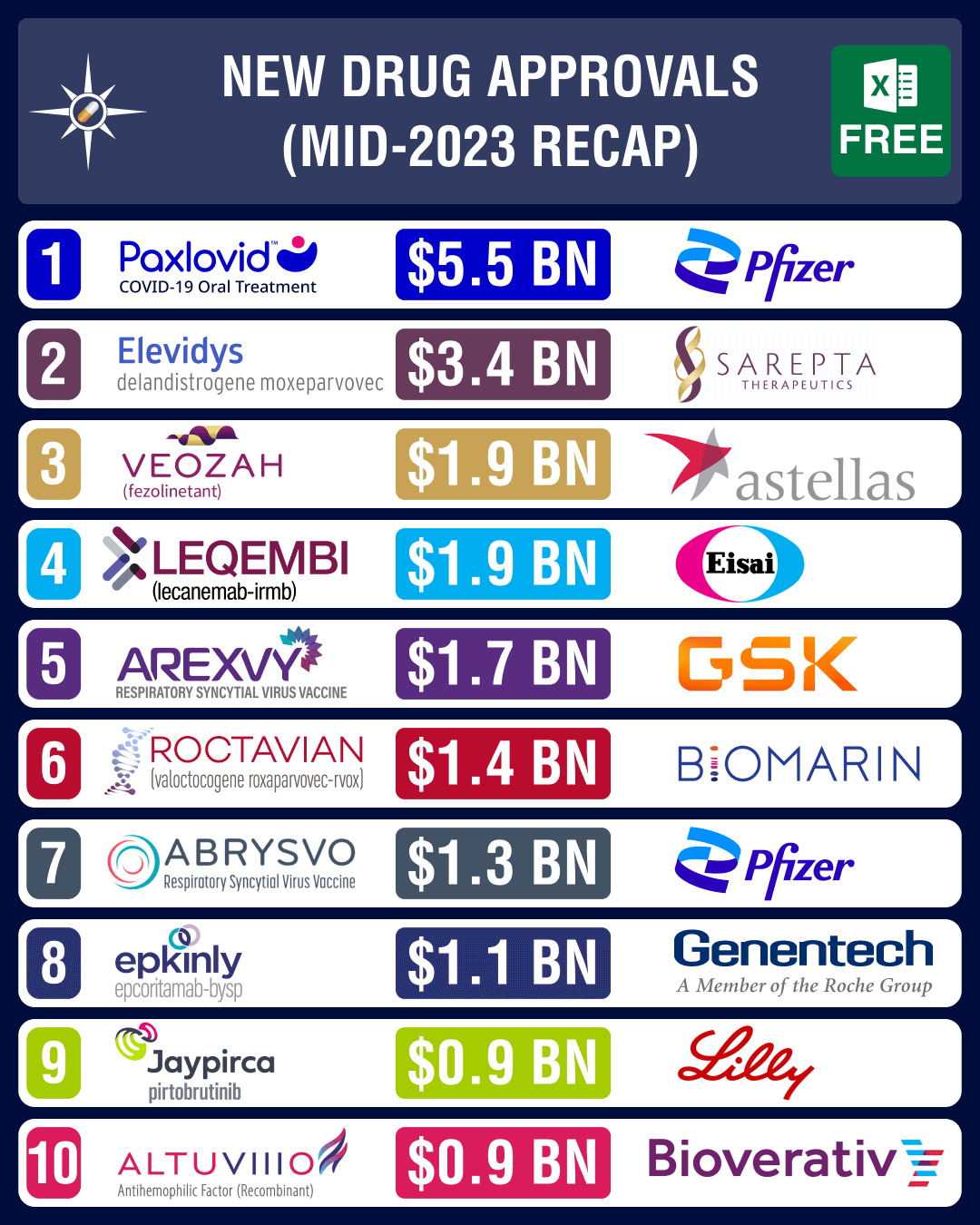


Closure
Thus, we hope this text has offered useful insights into Decoding the FDA Drug Approval Calendar 2024: A Potential Take a look at Potential Breakthroughs and Challenges. We thanks for taking the time to learn this text. See you in our subsequent article!
